

As mentioned before, they can be used to remove salts or specific ions depending on. In meteorology a precipitate is liquid or solid water (rain, snow, etc.) falling from the sky. Precipitation reactions are applied to many sample preparation processes.

Precipitates are insoluble salts that develop during precipitation reactions. Precipitate: In chemistry, a solid formed by a change in a solution, often due to a chemical reaction or change in temperature that decreases solubility of a solid. You can use ammonia solution instead of sodium hydroxide solution, but there are different results for aluminium and copper(II) salts when you use excess ammonia. Precipitate Meaning in Chemistry/Precipitation Reaction Definition: The word precipitation meaning refers to a chemical reaction formation that occurs in an aqueous solution and results in the creation of an insoluble salt when two ionic bonds join. It is the primary connection in the water cycle that. Two soluble salts react in solution leading to the formation of one or more insoluble products arecalled the precipitate.
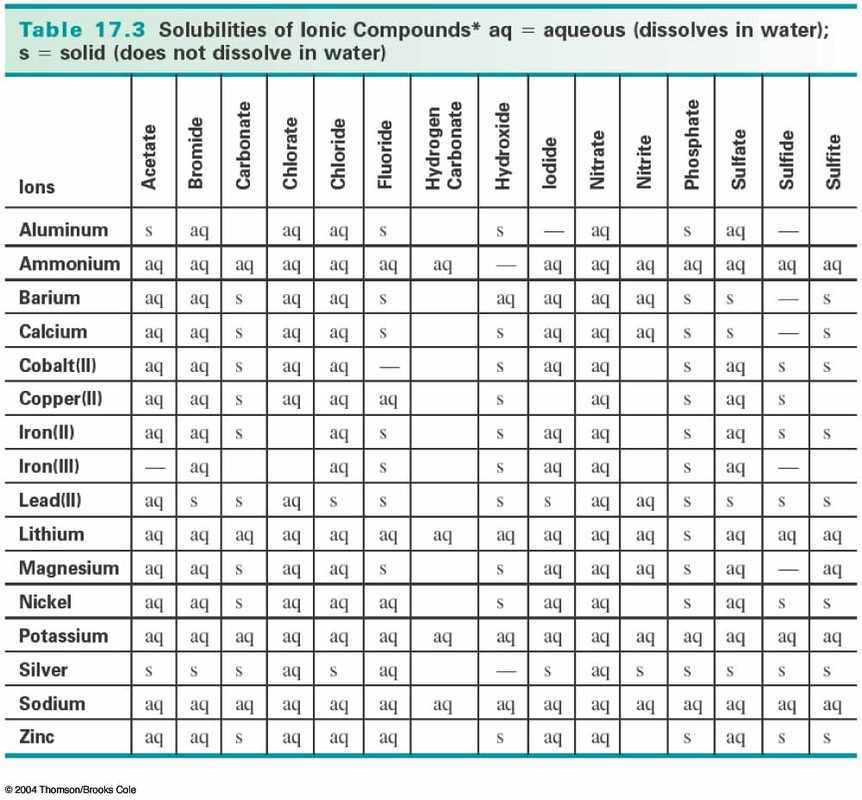
Solutions containing copper(II) ions form a blue precipitate when mixed with sodium hydroxide solution. Precipitation is water released from clouds in the form of rain, freezing rain, sleet, snow, or hail. Solutions containing copper(II) ions form a blue precipitate when mixed with sodium hydroxide solution Precipitation refers to a chemical reaction that occurs in aqueous solution when two ions bond together to form an insoluble salt, which is known as the precipitate. When metal ions combine with the hydroxide ions (OH-) from either sodium hydroxide solution or ammonia solution, they form insoluble precipitates. Precipitation reactions transform ions into an insoluble salt in aqueous solution.


 0 kommentar(er)
0 kommentar(er)
